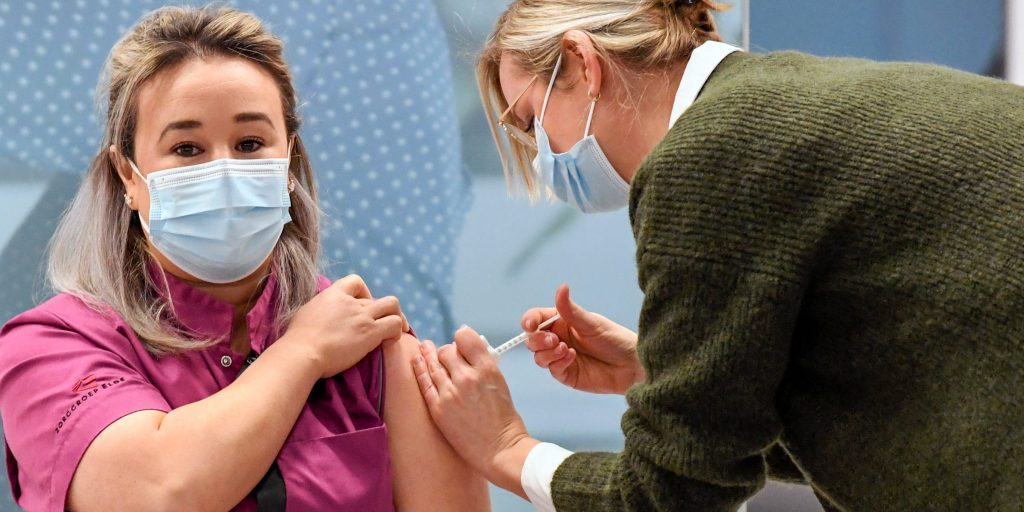
Piroschka van de Wouw/Pool via AP
- Mixing the AstraZeneca and Pfizer vaccines made regular side-effects worse, an Oxford study said.
- Some countries in Europe advise mixing doses after limiting the use of the AstraZeneca shot.
- The symptoms, though unpleasant, were not dangerous, a study author said.
- See more stories on Insider's business page.
People who mixed doses of the Pfizer and AstraZeneca vaccines get worse versions of the common, flu-like side effects, a UK study said.
Countries which are mixing and matching vaccines should prepared for more people having to take time off work after getting their second dose, the scientists said in a press release.
The findings came from early results of the COM-Cov study, which looks at the effects of mixing the Pfizer and AstraZeneca vaccines.
It showed that mild and moderate reactions to the vaccine, such as fatigue, headache, are more frequent if the vaccines are mixed versus getting two rounds of the same shot.
This was the case regardless of which vaccine came first.
Here are some of the reactions reported after the second dose of the vaccine:
- Feverishness (mild and moderate)- Pfizer/Pfizer: 21%, AstraZeneca/AstraZeneca: 10%, Pfizer/AstraZeneca: 41% (increase 21%), AstraZeneca/Pfizer: 34% (increase: 24%)
- Fatigue (mild and moderate)- Pfizer/Pfizer: 55%, AstraZeneca/AstraZeneca: 50%, Pfizer/AstraZeneca: 68% (increase 13%), AstraZeneca/Pfizer: 77% (increase 27%)
- Chills (mild and moderate)- Pfizer/Pfizer: 24%, AstraZeneca/AstraZeneca: 12%, Pfizer/AstraZeneca: 46%(increase 23%), AstraZeneca/Pfizer: 38% (increase: 26%)
Some countries, such as France, Germany, and the UK, recommend vaccine-mixing in some capacity.
Most often the advice is that younger people who had a first dose of the AstraZeneca vaccine should receive a different dose for their second. The policies come from concerns about a very rare blood clots associated with the AstraZeneca vaccine.
Describing the results, study author and Oxford professor Matthew Snape told reporters that the side-effects "are more or less the same type of reactions that we're seeing with the standard schedules, its just that they are occuring more frequently."
The symptoms went away within 48 hours, Snape said. None of the people in the study were hospitalized, the scientists said.
Snape said that a more fundamental question about vaccine-mixing - whether it gives better protection from COVID-19 - is still unknown.
One suggestion was that vaccine-mixing might lead to short-term practical problems, like people calling in sick to work.
"One of the things its telling us is, for example, is that you wouldn't want to immune a ward full of nurses with a mixed schedule because you may have higher rates of absentees in the next day," Snape told reporters.
Severe fatigue and malaise - side-effects serious enough to seek help from a doctor - increased slightly if the vaccine doses were mixed, from about 3% to about 10% for fatigue, Snape said.
But he noted that these are interim results of the study, and that no statistical analysis has been done on the numbers, so the figures should be taken with a grain of salt.
The study was run on participants over the age of 50, and the vaccines were given four weeks apart.
It is possible that the reactions to mixing the vaccines would be more severe in younger groups, which generally get more side-effects, Snape said.
The findings, published as a letter in the peer-reviewed journal The Lancet on Thursday, are the from the Com-COV study.
Future experiments are planned to test whether spacing out the doses to 12 weeks will change things.